Chemistry Form 4 Chapter 7 Acid and Base
An AP site apurinicapyrimidinic site also known as an abasic site. Time of Indicator Color Change sec Time at Equivalence Point sec PH at Equivalence Point.
Which of these statements are correct.
. The differences listed above depicts the clear difference between acids and bases which forms part of the chemistry and discussed among students the world over. Denoted is a quantitative measure of the strength of an acid in solutionIt is the equilibrium constant for a chemical reaction known as dissociation in the context of acidbase reactionsThe chemical species HA is an acid that dissociates into A the. 69 sec 104 sec 7 2 12.
As we will explore in Chapter 13 methylation of DNA also serves as an important mechanism regulating gene expression. Acid- Base Combinatio n. Another perhaps simpler way to predict the outcome of this reaction is to use the pK a values of the two acids CH 3 CO 2 H 48 and H 2 O 14 clearly acetic acid is a much stronger acid than water and therefore the equilibrium position for this reaction will lie over to the right in favor of the weakest acid and the weakest base.
Figure 127 Abasic Sites. As you find a seat in the classroom you read the. Solution 8 a Blue litmus will turn.
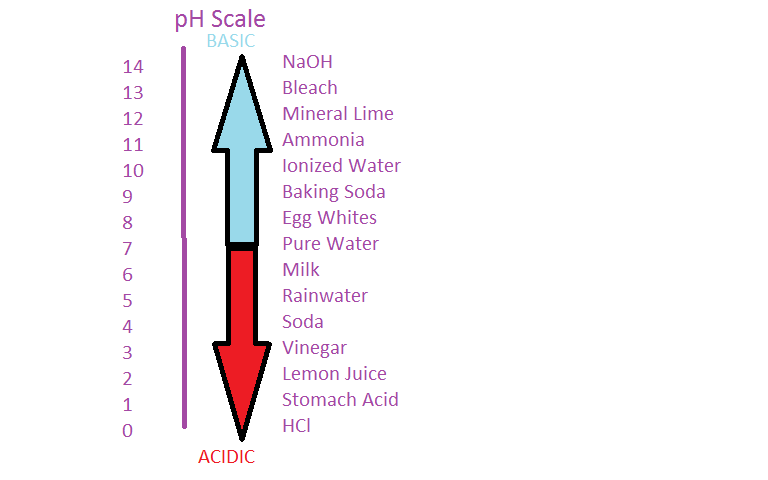
Initial PH Final PH. Acidity is indicated by a pH less than 7 while a pH greater than 7 indicates a base. Concise Chemistry Part II - Selina Solutions for Class Chemistry ICSE Chapter 3.
Class 11th Chemistry Chapter 1 notes cover all. Pingoud A Wilson GG and Wende W. D Change of colour in an acid and a base depends on the type of the indicator.
If section 46 were moved to chapter 12 then 56 and 75 would likely need to be moved into an organic or biological chemistry chapter as well. You stop to fill your cars gas tank almost making you late for the first day of chemistry class. Important topics covered in NCERT Solutions for class 7 chapter 5 Acids Bases and Salts.
The other sections that could fit within either a general or organicbiological chemistry chapter are sections 56 redox in organic and biochemistry and 75 energy of biochemical reactions. Apurinic and Apyrimidimic AP sites occur due to unstable hydrolysis. A base reacts with an acid to form a salt and water only.
Strong Acid Strong Base. I All four ii a and d iii b c and d iv only d. Iv Only d is correct.
PH is a measurement of the proportion of free hydrogen and hydroxyl ions in water. This type of reaction is known as neutralisation. X Y and Z are a general base a Lewis acid and a general acid respectively.
2014 Nuc Acids Res 42127489-7527. Type II restriction enzymes typically form a homodimer when binding with DNA as shown in the crystal structure of Bgl II in Figure 726B. In chemistry an acid dissociation constant also known as acidity constant or acid-ionization constant.
A table with data collected from the experiment consisting of things such as time initial and final pH. Get free access to Acids Bases and Salts Class Solutions which includes all the exercises with solved solutions. This is the base of Chemistry and what must be noted is that a lot of the important objectives - easy marks questions form the base of this chapter appear every year in Class 11 Chemistry Exam.

Determining Ph Of A Solution Acidic Basic Neutral Solutions Video Lesson Transcript Study Com



No comments for "Chemistry Form 4 Chapter 7 Acid and Base"
Post a Comment